Do the number of ao’s = number of mo’s? Draw the mo diagram for hf:

Molecular Orbital Diagrams Simplified By Megan Lim Medium
In homonuclear molecules, the atomic orbitals of two similar atom combine and a covalent bond is formed.

How to draw molecular orbital diagram for heteronuclear molecules. Now, mo diagrams are only simple for elements of the second row of the periodic table ($\ce{li}$ through $\ce{ne}$). You have now 2 electrons left,. It is a linear molecule.
Consider the h 2 molecule, for example. The lewis structure shows that the beryllium in beh 2 makes 2 bonds and has no lone pairs. These, however, also have their own depth;
Label each level with σ, σ*, π, π* In the construction of molecular orbital diagrams for heteronuclear molecules the bonding mos are shown closed to electronegative atoms while. Fill the mos with electrons:
You have to start filling the orbitals from those with lowest energy to those with higher energy. So, 2 electrons on σ2s , two electrons on σ∗2s, two electrons on σ2p. Orbital interactions that produce bonding or antibonding orbitals in heteronuclear diatomics occur if there is sufficient overlap between atomic orbitals, as determined by their symmetries and similarity in orbital energies.
Fill the mos with electrons: There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. Molecular orbital theory mot chemistry study material.
Do the number of ao’s = number of mo’s? Where c1 and c2 are two constants having different values for different atoms. Sparknotes organic chemistry orbitals problems molecular orbital.
Drawing atomic and molecular orbitals diagrams for molecules. Molecular orbital diagram of lih. The molecular orbitals which describe the motion of a single electron in a molecule containing two unequal nuclear charges will not exhibit the g and u symmetry properties of the homonuclear diatomic case.
Another orbital is formed by subtracting one of these functions. Week 9 day 2 ch 7 pt 3. Valence bond theory and molecular.
The drawing of the mo diagram of heteronuclear molecules. “f” will be lower on the diagram. Use the buttons at the top of the tool to add % (8).
Construct the molecular orbital diagram for he2. General notes on molecular orbital diagrams. To make sense of the complexities introduced by antibonding, we build molecular orbital diagrams.
Involving heavier atoms makes it harder to guess at molecular orbital diagrams, and there is need for quantum chemistry calculations. $\begingroup$ muliken's 1935 electronic structures of polyatomic molecules. “f” will be lower on the diagram.
3) combine salcs with orbitals on a for more complex molecules, there may be more than 2 symmetry equivalent types of atoms. One of the molecular orbitals in this molecule is constructed by adding the mathematical functions for the two 1s atomic orbitals that come together to form this molecule. The other is for after nitrogen (start.
A molecular orbital diagram for a diatomic molecule (two atoms) always has the same basic pattern. Also the molecular orbitals formed are unsymmetrical due to difference in electronegativities. This is the general mo diagram you need to fill with the valence electrons of bn boron has 3 valence electrons, and nitrogen has 5 valence electrons, this makes 8 electrons.
Co in molecules with more than one type of atom, mos are formed from aos that have different energies. Ψmo = c1 ψ1 ao + c2 ψ2 ao. 8 4 molecular orbital theory chemistry.
Molecular orbitals are obtained by combining the atomic orbitals on the atoms in the molecule. And 2p orbitals, but that is not how sodium chloride is made. We shall consider the molecular orbitals in lih, ch and hf to illustrate how molecular orbital theory describes the bonding in heteronuclear molecules, and to.
Sodium atoms are construct an mo diagram for lih and suggest what type of bond it might have. Overlapping atomic orbitals produce molecular orbitals located in the middle of the diagram. In this case, mo diagrams can be constructed from the orbitals of two chemically reasonable fragments.
Draw a picture of the levels. Individual atomic orbitals (ao) are arranged on the far left and far right of the diagram. Molecular orbital diagram unmasa dalha.
Examples of heteronuclear diatomic molecules. Molecular orbitals for heteronuclear molecules. There are two mo diagrams you need to memorize for diatoms (n2, o2, ne2, etc).one is for the elements up to nitrogen.
The molecular orbitals in the heteronuclear case will in general be concentrated more around one nucleus than the other. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories:
In hydrogen fluoride, hf, symmetry allows for overlap between the h 1s and f 2s orbitals, but the difference. The further to the right your element is, the lower its energy levels are. Sp hybrid orbitals in beh2 1.
N2 = (he) 2s2 2p3 (5 valence electrons for each atom). (such as h2o, nh3, and ch4.)

Draw And Completely Label The Molecular Orbital Energy Level Diagram For The Heteronuclear Diatomic Molecule No Assume No Has Orbitals Like O2 Show Only The Valence Orbitals 2s And 2p Studycom
What Is The Molecular Orbital Diagram For Hcl - Quora
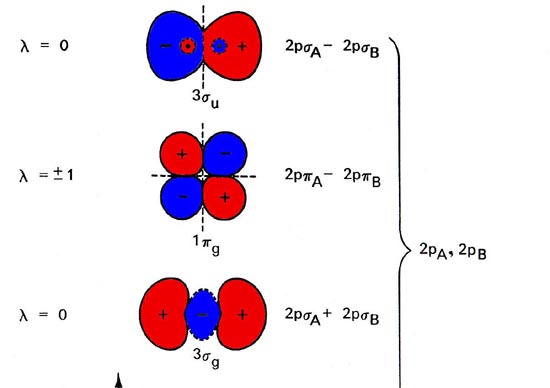
Molecular Orbitals - Molecular Orbitals For Homonuclear Diatomics

Molecular Orbital Diagram - Wikiwand

Solved Draw A Molecular Orbital Diagram For Each Of The Cheggcom
Molecular Orbitals Introductory Chemistry 1st Canadian Edition Clone
Molecular Orbitals Molecular Orbital Theory Sparknotes
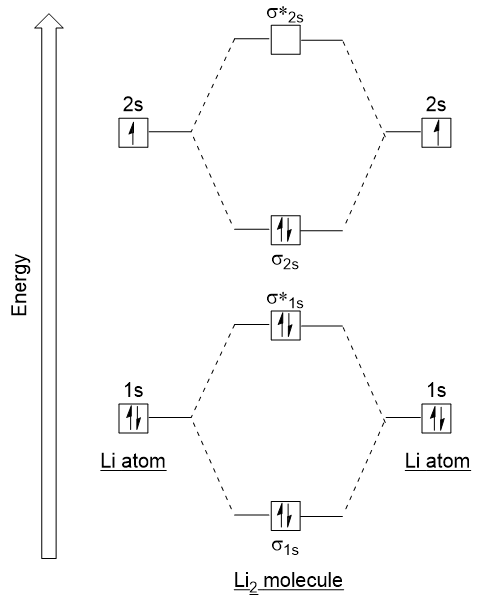
Molecular Orbitals Introductory Chemistry 1st Canadian Edition

Solved Chapter 11 Problem 39e Solution Selected Solutions Manual -- General Chemistry 10th Edition Cheggcom
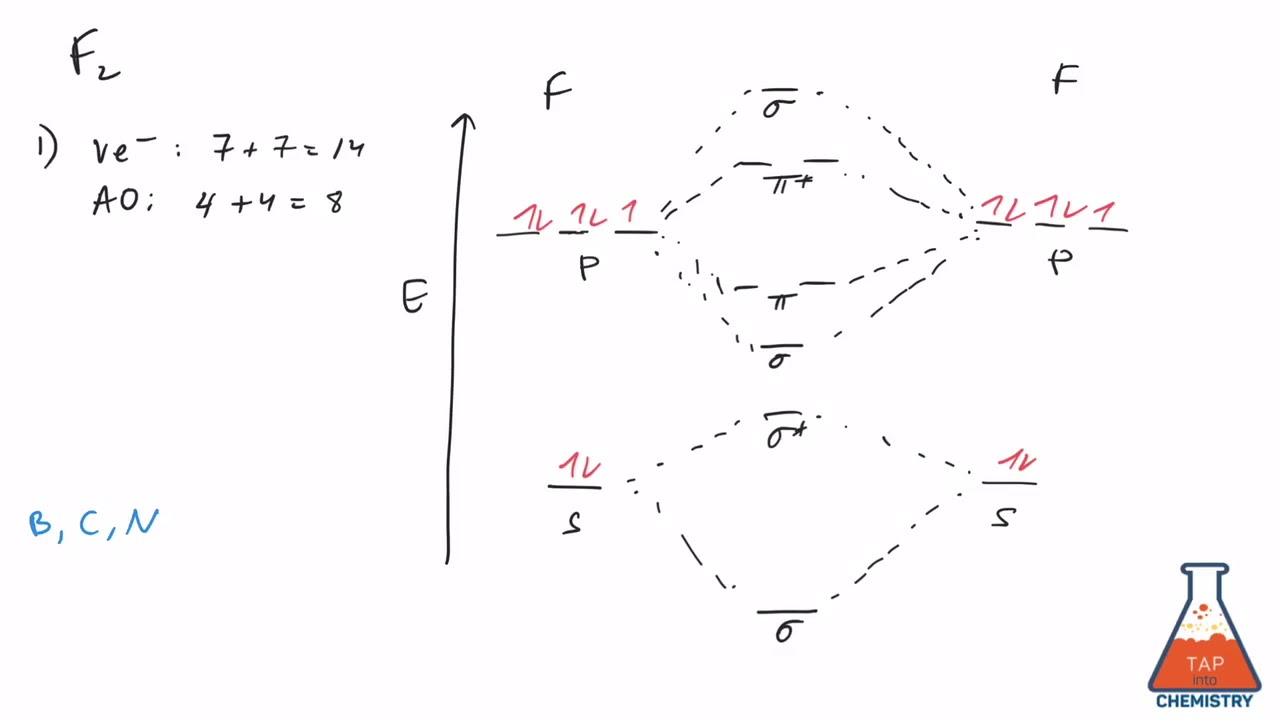
Mo Diagram 2 - F2 - Youtube

Molecular Orbital Diagrams Simplified By Megan Lim Medium
Molecular Orbitals Molecular Orbital Theory Sparknotes

Molecular Orbital Diagram Of Polyatomic Co2 Molecules - Chemical Bonding Molecular Structures - Youtube
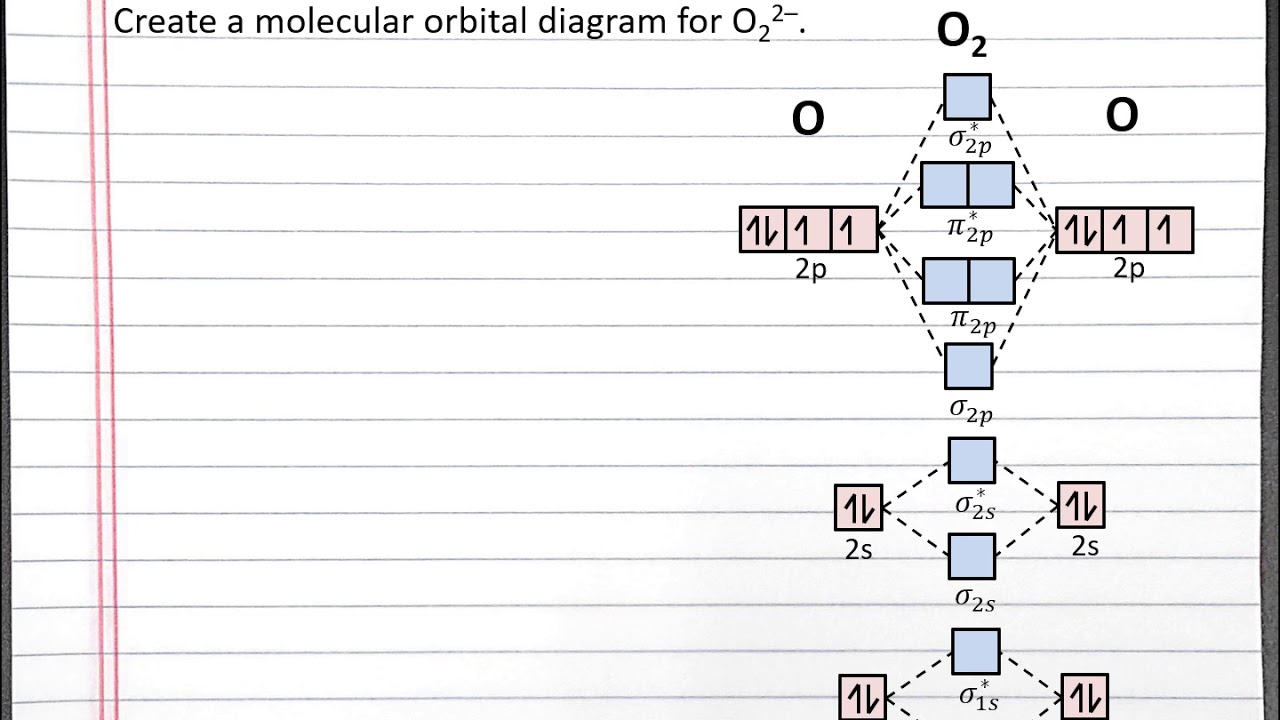
Chem 101 - Creating A Molecular Orbital Diagram For A Diatomic Ion In The Second Row With Aleks - Youtube

Draw The Mo Diagram For Cn- And Identify T Clutch Prep
How Do We Draw The Molecular Orbital Diagram Of Bf - Quora

Molecular Orbital Theory Heteronuclear Diatomic Cyanide Cn- Example - Youtube
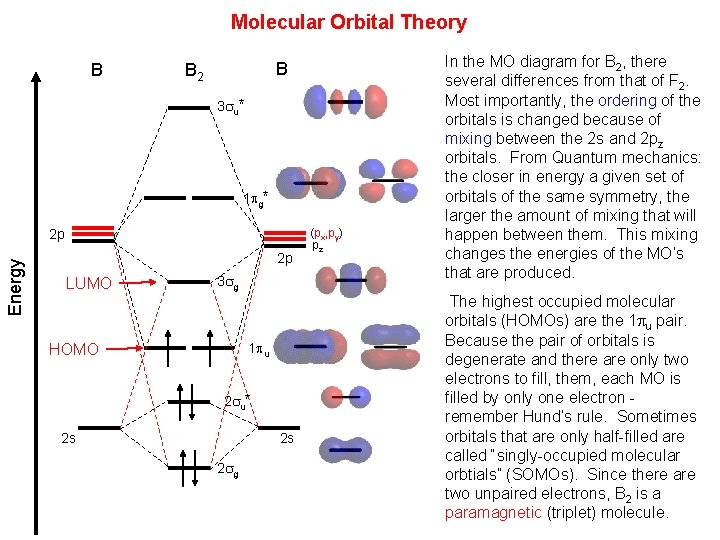
Molecular Orbital Theory Or When Electrons Dont Like
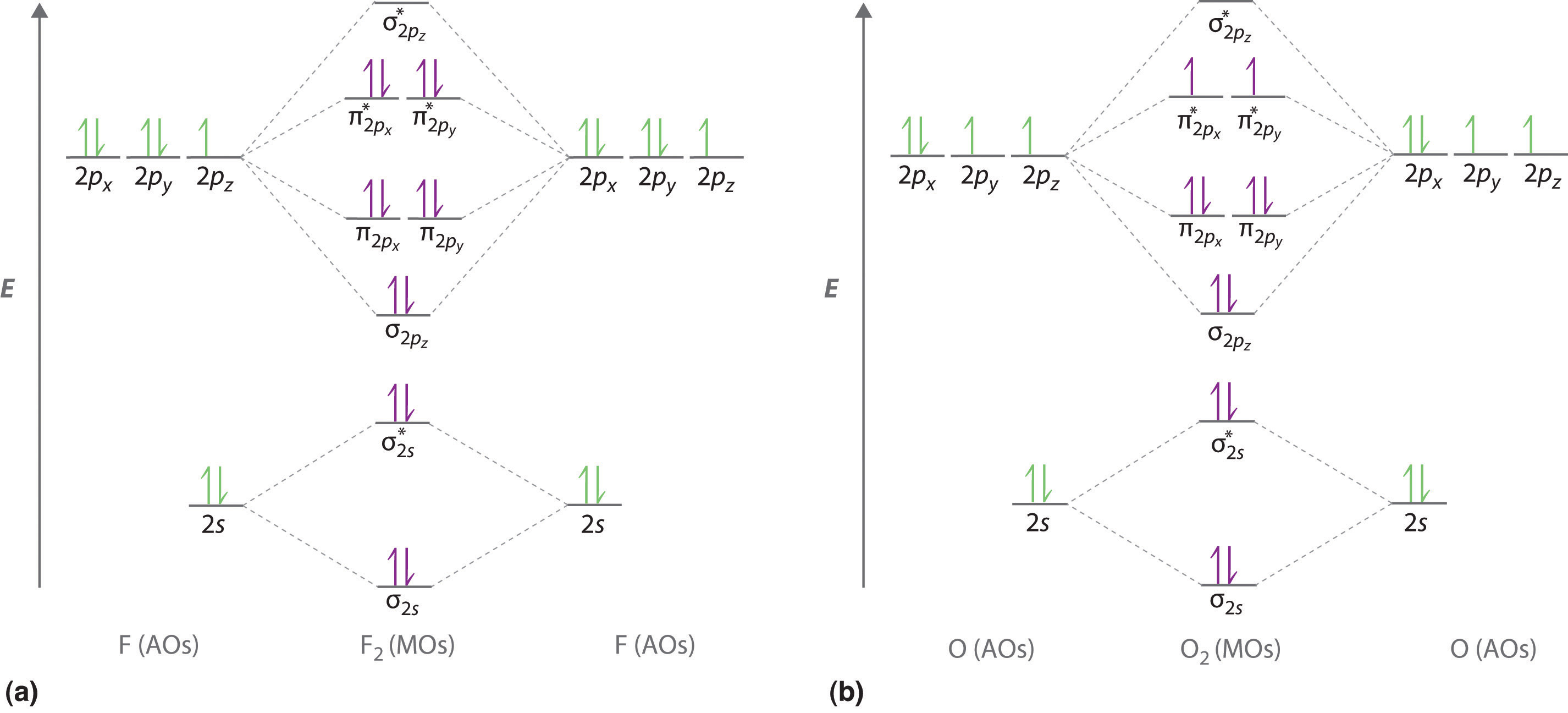
98 Mo Theory And The Period 2 Diatomic Molecules - Chemistry Libretexts